Product Description:
- Name: EGFR Gene Mutation Detection Kit
- Purpose: Qualitative detection of 11 common mutations in the human EGFR gene.
- Specimens: DNA specimens from paraffin-embedded sections of patients clinically diagnosed with non-small cell lung cancer.
- Verification: The product has been verified for the detection performance of target gene mutations but has not been combined with specific drugs in clinical trials.
Use and Limitations:
- Use: Detection results can assist in screening suitable non-small cell lung cancer patients for EGFR-targeted therapy.
- Limitations: Detection results alone cannot be used as the sole basis for clinical diagnosis.
Background Information:
- EGFR: Epidermal Growth Factor Receptor, a membrane protein crucial for cell proliferation, growth, repair, and tumor cell survival.
- Gene Structure: Located on chromosome 7 (7p12~14), the EGFR gene comprises 28 exons. Exons 18-24 form the tyrosine kinase domain encoding the gene.
- Mutation Focus: Most mutations occur in exons 18-21, notably exon 19 deletion and exon 21 L858R mutation.
- Clinical Relevance: These mutations predict the efficacy of targeted drugs in treating non-small cell lung cancer and guide tumor treatment drug selection.
- FDA Requirement: EGFR gene mutation detection is mandated for patients considering EGFR-TKI treatment.
Table 1: Common Mutations of EGFR Gene
| Mutation | Exon | Changes of amino acid sequence | Changes of base sequence | Cosmic ID |
|---|---|---|---|---|
| Ex 18-Mu-1 | 18 | G719S | 2155G>A | 6252 |
| Ex 18-Mu-2 | 18 | G719C | 2155G>T | 6253 |
| Ex 18-Mu-3 | 18 | G719A | 2156G>C | 6239 |
| Ex 19-Mu-1 | 19 | Glu746-Ala750del | 2235-2249del | 6223 |
| Ex 20-Mu-1 | 20 | S768I | 2303G>T | 6241 |
| Ex 20-Mu-2 | 20 | T790M | 2369C>T | 6240 |
| Ex 20-Mu-3 | 20 | V769-D770insASV | 2307_2308insGCCAGCGTG | 12376 |
| Ex 20-Mu-4 | 20 | H773-V774insH | 2319_2320insCAC | 12377 |
| Ex 20-Mu-5 | 20 | D770-N771insG | 2310_2311insGGT | 12378 |
| Ex 21-Mu-1 | 21 | L858R | 2573T>G | 6224 |
| Ex 21-Mu-2 | 21 | L861Q | 2582T>A | 6213 |
Test Principle
This kit is designed to detect specific mutations in the EGFR gene. It includes primers and probes for detecting:
- G719X mutations (G719S, G719C, G719A) in exon 18,
- Deletion mutations of exon 19 (including 2235-2249del 15),
- S768I and T790M mutations in exon 20,
- Three types of insertion mutations (2307_2308insGCCAGCGTG, 2319_2320insCAC, and 2310_2311insGGT),
- L858R and L861Q mutations in exon 21.
During mutation detection, if the sample contains mutational DNA templates:
- The primers and probes specific to the detected mutation will bind to the template DNA.
- PCR amplification will occur, releasing fluorescence signals.
- Real-time monitoring and output of fluorescence signal strength will be conducted using a fluorescent quantitative PCR amplifier.
If the sample does not contain mutational DNA templates:
- The primers and probes specific to the mutation will not bind to the template DNA, or their binding will be blocked.
- This will result in no PCR amplification or inhibited PCR amplification.
- Consequently, no fluorescence signals will be released.
Overall, the kit enables qualitative analysis of detection results through real-time monitoring of fluorescence signals during PCR amplification.
Table 2 Main Ingredients of the Kit
| Tube No. | Label name | Main ingredients | Packing specifications (12 tests/kit) | Packing specifications (24 tests/kit) |
|---|---|---|---|---|
| 1 | G719X PCR reaction liquid | PCR buffer, dNTPs, G719X primer and probe | 1 tube (290µL) | 1 tube (580µL) |
| 2 | S768I PCR reaction liquid | PCR buffer, dNTPs, S768I primer and probe | 1 tube (290µL) | 1 tube (580µL) |
| 3 | T790M PCR reaction liquid | PCR buffer, dNTPs, T790M primer and probe | 1 tube (290µL) | 1 tube (580µL) |
| 4 | L858R PCR reaction liquid | PCR buffer, dNTPs, L858R primer and probe | 1 tube (290µL) | 1 tube (580µL) |
| 5 | L861Q PCR reaction liquid | PCR buffer, dNTPs, L861Q primer and probe | 1 tube (290µL) | 1 tube (580µL) |
| 6 | Ins PCR reaction liquid | PCR buffer, dNTPs, Ins primer and probe | 1 tube (290µL) | 1 tube (580µL) |
| 7 | Del PCR reaction liquid | PCR buffer, dNTPs, Del primer and probe | 1 tube (290µL) | 1 tube (580µL) |
| 8 | Quality control PCR reaction liquid | PCR buffer, dNTPs, quality control primer and probe | 1 tube (290µL) | 1 tube (580µL) |
| 9 | Enzyme mixture | Enzyme mixture containing Taq enzyme and UNG enzyme | 1 tube (24µL) | 1 tube (48µL) |
| 10 | Reference dye | ROX dye | 1 tube (45µL) | 1 tube (90µL) |
| 11 | Internal standard template | Internal standard DNA template | 1 tube (100µL) | 1 tube (100µL) |
| 12 | Weakly positive control | Containing mutation DNA template | 1 tube (200µL) | 1 tube (200µL) |
| 13 | Blank control | Tris-HCl buffer (10mM) | 1 tube (200µL) | 1 tube (200µL) |
Storage Conditions and Shelf Life:
- The shelf life of the kit is 9 months when stored at -20℃±5℃ and protected from light.
- Minimize freezing and thawing to less than 6 times during use.
- Production date and expiry date are indicated on the label.
Applicable Instruments:
- The kit is compatible with Stratagene MX3000P, AB 7500, and LightCycler 480 real-time fluorescent quantitative PCR amplifiers.
- Use the FAM channel to collect fluorescent signals of EGFR gene amplification, VIC/JOE/HEX channel for internal standard gene amplification signals, and set up the reference signal (ROX).
Specimen Requirements:
- High-quality DNA is crucial. The kit is suitable for detecting human genome DNA extracted from paraffin-embedded pathological tissues confirmed by a doctor to contain tumor tissues. Specimens within 3 years are suggested.
- QIAGEN DNA Extraction Kit (QIAamp DNA FFPE Tissue Kit, Cat No. 56404) is recommended. Extracted DNA concentration and purity should be tested with a UV spectrophotometer (OD260/OD280: 1.8-2.0). Dilute DNA to 10-15ng/μL and test immediately or store below -20℃ without repetitive freezing and thawing.
Test Methods:
1. Reagent Preparation:
- Allow the kit to reach room temperature, fully melt all components, and centrifuge for 10 seconds.
- Determine the number of reactions (n) needed. Prepare 7 types of mutation PCR reaction mixtures and quality control PCR reaction mixtures according to the specified method in Table 3.
- n=Number of specimens+Blank control (1T)+Weakly positive control (1T)
- Pack the prepared tube 1-8 PCR reaction liquid into reaction tubes according to the packing amount of 23μL per well.
- Transfer PCR reaction tubes to the specimen preparation area. Store the remaining reagent back in the refrigerator at -20℃ ±5℃ for freezing and protection from light.
Table 3: Preparation Method of Each PCR Reaction System
| Component | Formula of single reaction system | Formula of n reaction systems |
|---|---|---|
| PCR reaction liquid | 22.3μL | 22.3×n μL |
| Enzyme mixture | 0.2μL | 0.2×n μL |
| Reference dye (ROX) | 0.4μL | 0.4×n μL |
| Internal standard template | 0.1μL | 0.1×n μL |
2. Specimen Preparation:
(Specimen preparation area)
- Extraction of Specimen DNA:
- Follow the instructions provided with the purchased commercial genome DNA extraction kit.
- Sample Adding:
- a) Add genome DNA, weakly positive control, and blank control of the specimens to be tested into the reaction tubes containing the 1-8 PCR reaction mixture. Each specimen is tested with 8 types of PCR reaction mixture, with an additional amount of 2μL per well. The recommended concentration of specimen DNA is 10-15ng/μL.
- b) If the PCR reaction tubes with added templates cannot be immediately placed onto the instrument due to temporary situations, store them at 2-8℃ temporarily and conduct detection on the instrument as soon as possible within 24 hours.
3. PCR Amplification:
(Nucleic acid amplification area)
- Start the instrument and perform self-inspection of instrument performance.
- Take the PCR reaction tubes prepared in the specimen preparation area and place them in corresponding positions in the sample tank of the instrument. Record the placement sequence.
- Set up relevant parameters for nucleic acid amplification according to Table 4 and start PCR amplification. After the reaction is completed, define an appropriate fluorescence threshold (generally delimited in the middle of the exponential growth phase under the logarithmic form of the amplification curve) to obtain Ct values for different channels and calculate relevant △Ct values according to the amplification curve.
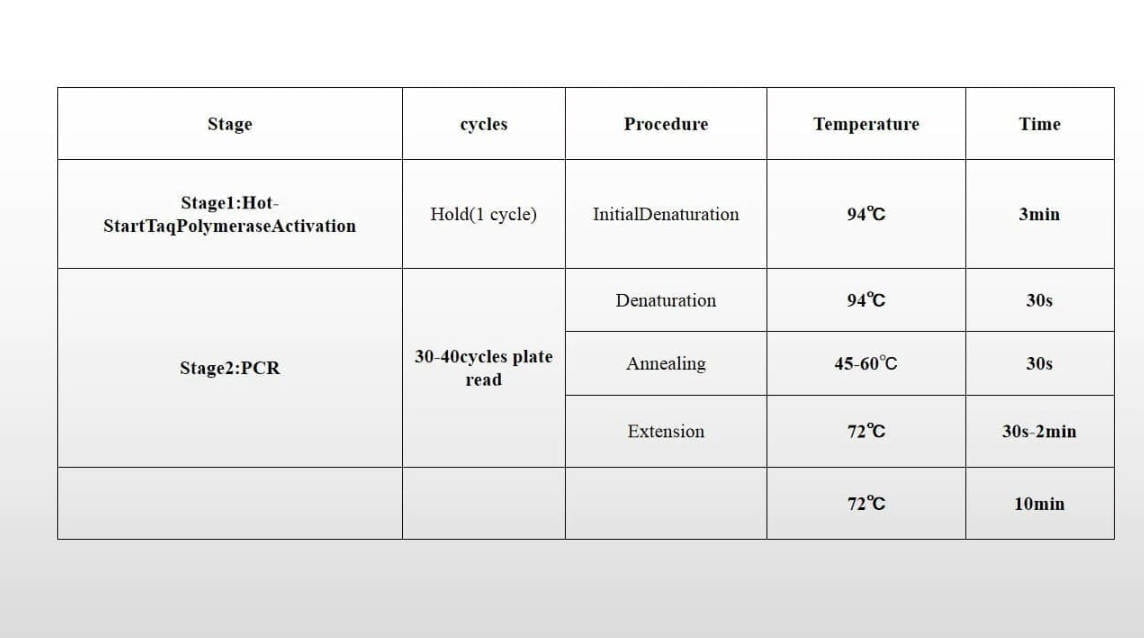
Table 4: Relevant Parameters of Nucleic Acid Amplification of Instrument
| PCR Reaction Conditions | Phase | Conditions | Number of cycles |
|---|---|---|---|
| UNG treatment | 37℃ | 10 minutes (min) | 1 |
| Pre-degeneration | 95℃ | 5 minutes (min) | 1 |
| PCR | 95℃ | 15 seconds (s) | 40 |
| 60℃ | 60 seconds (s) | ||
| (Fluorescence signals collected at the end of this phase) |
Signal Acquisition:
- EGFR gene: Fluorescence signals are collected from the FAM channel.
- Internal standard gene: Fluorescence signals are collected from the HEX/VIC/JOE channel.
Result Analysis:
After the completion of the reaction, the threshold is set according to the amplification curve to calculate △Ct (△Ct = Mutation detection Ct – Quality control detection Ct) and interpret the results.
Interpretation of Test Results:
1. Determination of Kit Effectiveness:
- Weakly positive control:
- Ct value of FAM channel is ≤32 with an obvious exponential growth phase in the amplification curve.
- Blank control:
- FAM channel shows no amplification curve or a straight line or slight oblique line. No obvious exponential growth phase, Ct value is ≥38.
- Internal standard gene:
- In blank control, the Ct value of HEX/VIC/JOE channel is <38 with an obvious exponential growth phase.
- In the tested specimen and weakly positive reaction tube, occasional absence of signal in the HEX/VIC/JOE channel when the FAM channel has a signal may occur due to internal standard amplification inhibition by the target gene amplification.
2. Determination of Specimen Effectiveness:
- Quality control PCR reaction liquid:
- For FAM channel, Ct < 23, indicating specimen genome DNA overdose. Suggested re-testing after dilution.
- Quality control PCR reaction liquid:
- For FAM channel, 23 ≤ Ct ≤ 30, indicating moderate dose of specimen genome DNA.
- Quality control PCR reaction liquid:
- For FAM channel, 30 < Ct < 34, indicating lower dose of specimen genome DNA. Mutation type can be tested only in specimens with higher content of mutation DNA.
- Quality control PCR reaction liquid:
- For FAM channel, Ct ≥ 34, indicating too low adding dose of specimen genome DNA. Requires preparation of new specimen or addition of usage amount for detection.
3. Determination of Detection Results:
After determining the effectiveness of detection, calculate the △Ct value of effective detection specimens and determine the detection results according to the following steps and Table 5 to confirm the presence of mutation in the specimen.
- In specimen mutation detection, Ct value > 36 indicates the specimen is negative or below the lower detection limit of this kit.
- In specimen mutation detection, if Ct value is ≤ 36, calculate the △Ct value of this reaction tube. If △Ct value is less than the corresponding △Ct Cut-Off value, the specimen is considered mutation-positive. Otherwise, it is considered mutation negative or below the lower detection limit of this kit.
- Applying the above determination criteria, the mutation type of G719X positive specimen may be one of G719S, G719C, and G719A. The mutation type of Ins positive specimen may be one of V769-D770insASV, H773-V774insH, and D770-N771insG. This product cannot be further divided.
Table 5: Determination of Results
| Reaction Liquid | Mutation Type | Negative | Positive |
|---|---|---|---|
| G719X PCR reaction liquid | G719X | Ct > 36 or no Ct value | Ct ≤ 36, ΔCt ≥ 7 |
| S768I PCR reaction liquid | S768I | Ct > 36 or no Ct value | Ct ≤ 36, ΔCt ≥ 7 |
| T790M PCR reaction liquid | T790M | Ct > 36 or no Ct value | Ct ≤ 36, ΔCt ≥ 7 |
| L858R PCR reaction liquid | L858R | Ct > 36 or no Ct value | Ct ≤ 36, ΔCt ≥ 7 |
| L861Q PCR reaction liquid | L861Q | Ct > 36 or no Ct value | Ct ≤ 36, ΔCt ≥ 7 |
| Ins PCR reaction liquid | Ins | Ct > 36 or no Ct value | Ct ≤ 36, ΔCt ≥ 6 |
| Del PCR reaction liquid | Del | Ct > 36 or no Ct value | Ct ≤ 36, ΔCt ≥ 6 |
Limitations of Test Methods:
- The accuracy of specimen detection results depends on the quality of specimen collection, treatment, and storage. Errors in these processes can lead to inaccurate detection results. Poor DNA quality during specimen treatment may result in false negative results. For the FAM channel of the quality control PCR reaction liquid, specimens with Ct values between 23 and 30 indicate relatively good DNA quality and moderate sample addition, resulting in the most accurate detection results.
- Negative results obtained from specimen detection cannot rule out the presence of mutations with low abundance or mutations in other loci of the EGFR gene.
- The detection results of this kit are intended for clinical reference to guide personalized medicine and should not be used as the sole basis for clinical diagnosis.
Product Performance Indexes:
- The kit is fully packed without overflow of contents. The label is intact with clear identification content. The composition of the kit is correct without repetition or missing components.
- Specific reference is detected using the 7 types of mutation reaction liquid included in the kit, and all detection results are negative.
- The sensitivity of corresponding mutations is detected using the 7 types of mutation reaction liquid included in the kit, and all detection results are positive.
- The lowest limit of detection can reach 1%, allowing the detection of various types of EGFR gene mutations in 20ng genome DNA.
- The kit is used to repeatedly detect the precision reference of the EGFR gene 10 times, with an intra-assay coefficient of variation (CV) of ≤5%.
Precautions:
- This kit is intended for in vitro diagnosis only.
- Do not mix reagents from this kit with those from other lots.
- The weakly positive control in this kit is not contagious but should be handled as potentially infectious material.
- Residual reagents in the kit and experiment wastes should be handled according to medical waste disposal guidelines.
References:
- NCCN Clinical Practice Guidelines in Oncology – Non-Small Cell Lung Cancer Guideline (Chinese edition), V1.2009.
- Huang Jie, Qu Shoufang, et al. Establishment of human EGFR gene mutation control materials. Chinese Journal of Pharmaceutical Analysis, 2011, 31(9):1758-1763.
- Expert Consensus on Gene Mutation Detection of Epidermal Growth Factor Receptor of Patients with Non-Small Cell Lung Cancer in China. Chinese Journal of Pathology, 2011, 40(10).







Reviews
There are no reviews yet.