Arrière-plan
Strawberries are a type of perennial herbaceous plant in the Rose family (Rosaceae) and the Strawberry genus (Fragaria). They have juicy texture, are nutritionally rich, widely cultivated, and can be eaten fresh or processed. Strawberries are an important economic crop.
Virus detection is a crucial step in preventing and controlling viral diseases and producing virus-free seedlings. Currently, there is no effective method to cure viral diseases in plants. Once a plant in the field is infected with a virus, it remains infected for its entire life and cannot be cured. Donc, virus detection plays a significant role in the sustainable development of the strawberry industry.
Introduction
There are more than 30 reported viruses that can infect strawberries. Common ones include Strawberry Vein Banding Virus (SVBV), Strawberry Mottle Virus (SMoV), Strawberry Crinkle Virus (SCV), and Strawberry Mild Yellow Edge Virus (SMYEV). The detection rate of SCV is relatively low in China. The continuous upgrade of detection technology and the expansion of cultivation areas lead to the discovery of new virus strains and types in strawberries. Strawberry Pallidosis-Associated Virus (SPaV) is found in Australia, Europe, and America.
Performing specific detection for only one virus at a time using a single RAP test is time-consuming and requires a lot of materials. Donc, establishing a multiplex PCR detection method that can simultaneously test for multiple viruses is important.
Multiplex PCR involves adding multiple pairs of primers in one PCR reaction to amplify multiple templates, allowing the simultaneous detection of various viruses. Multiplex PCR is more efficient and economical compared to single PCR. Establishing a stable multiplex PCR reaction system is challenging due to the interactions between primers, competitive amplification, and different optimal amplification conditions for various templates. It requires careful selection and optimization of specific primers, reaction conditions, and reaction systems. Multiplex PCR is not a simple mixture of single PCRs; the design of primers is a crucial factor determining the success of multiplex amplification, and there needs to be a difference in the sizes of multiple bands for effective gel electrophoresis differentiation.
Total RNA Extraction:
Pour extracting total RNA from strawberry leaves, the SEON Biotech Kit d'extraction d'ARN is used, following the instructions provided.
Reverse Transcription:
The reverse transcription process involves using M-MuLV Reverse Transcriptase from Takara (Chine) to synthesize cDNA.
The reaction system includes:
- ARN: 2 µL
- 5× M-MuLV Buffer (Promega): 4 µL
- dNTP Mix (2.5 mmol): 1 µL
- Random primers (10 μmol): 0.5 µL
- Oligo (dT)18 primers (10 μmol): 0.5 µL
- M-MuLV Reverse Transcriptase and RNase Inhibitor each: 0.5 µL
- RNase-free water to make up the system to 20 µL
- After thorough mixing, the solution is centrifuged briefly and then incubated at 37°C for 1 heure, followed by storage at -20°C.
Based on the gene sequence information from NCBI for SVBV, SMoV, SMYEV, SPaV, StrV-1, and BrYV, obtained from GenBank Accession Numbers FM867860.1, AJ311876.1, D12517.1, MN747002.1, MW795715, and ON060762, respectivement, conserved regions are identified using PrimerPremier 6.0 for primer design. The primer length ranges from 18 à 25 pb, and the GC content is between 40% et 60%.

Analyse
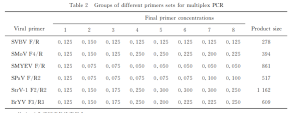
Multiple pairs of primers were designed and screened for SVBV, SMoV, SMYEV, SPaV, StrV-1, and BrYV. Initialement, the primer concentrations for all combinations were set at 0.125 μmol/L. The PCR amplification program was consistent with single PCR. After screening, a primer combination capable of simultaneously detecting six viruses was identified.
When the concentrations of all six primers were set at 0.125 μmol/L, SVBV bands were clear, SMYEV bands were the widest and brightest, SPaV bands were very bright, while SMoV, BrYV, and StrV-1 bands were weak. By adjusting the primer quantities for SMoV, BrYV, and StrV-1 while reducing the amounts for SMYEV and SPaV, a suitable primer ratio was determined: SVBV:SMoV:SMYEV:SPaV:BrYV: StrV-1 = 5:10:2:3:10:10.
Conclusion
In multiplex PCR, optimizing annealing temperature, annealing time, extension temperature, and extension time can reduce mutual interference between primers. In this study, annealing time (30-45 s) and extension temperature (68-72°C) had minimal impact on the multiplex reaction. Cependant, annealing temperature (50-60°C) and extension time (75-120 s) were crucial for the established reaction system. Too high an annealing temperature could lead to the failure of certain virus amplifications, likely related to primer annealing temperature and stability of primer-template binding. Both too-short and too-long extension times were unfavorable for the simultaneous amplification of the six viruses, affecting product yield and increasing the probability of nonspecific amplification. The multiplex PCR established in this experiment can identify SVBV, SMoV, SMYEV, SPaV, StrV-1, and BrYV in a single reaction, saving both cost and time in detection.