- Components of the reagent kit
| Tehnički podaci | 50T | 100T |
| Mačka. Ne. | SN0201 | SN0202 |
| DNA Extraction Columns (set) | 50 (set) | 100 (set) |
| Reagent Buffer Solution A | 30 ml | 2 × 30 ml |
| Reagent Buffer Solution C | 30 ml | 2 × 30 ml |
| Wash Buffer 1 | 15 ml | 2 × 15 ml |
| RNaza A | 1ml | 1ml |
| Elucijski pufer | 20 ml | 20 ml |
| Instruction Manual | 1 | 1 |
- Skladištenje
This kit should be stored at room temperature (15-25℃) in dry conditions and can be stored for 12 mjeseca. DNA extraction purification columns can be stored in a cool and dry environment for up to 1 year. RNase A contains a preservative and can be transported at room temperature, but for long-term storage, it should be kept at -20℃.
- Instructions for Using the Reagent Kit
3.1 This kit is intended for molecular biology research purposes and should not be used for disease diagnosis or treatment.
3.2 Some components in the kit contain irritants; it is advisable to take necessary precautions (such as wearing protective clothing and goggles).
3.3 The use of this kit requires additional equipment such as a high-speed centrifuge, water bath (metal bath), vortex mixer, anhydrous ethanol, liquid nitrogen, chloroform, sterile deionized water, and EP tubes.
- Introduction to the Reagent Kit
The CTAB-based Plant Pročišćavanje DNK Kit provides an improved CTAB method for purifying DNA, utilizing a specific binding buffer that efficiently precipitates DNA, and subsequently collects high-purity DNA through an adsorption column.
This kit is widely used for plant tissues and fungi, capable of extracting total DNA from samples within 2 sati (including mitochondrial DNA and chloroplast DNA). The extracted DNA can be directly used for downstream experiments such as PCR, Southern blotting, and others.
- Experimental Principles and Procedures
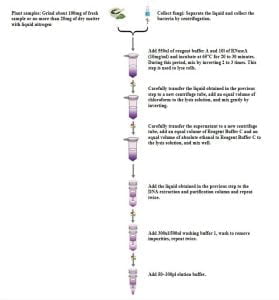
- Extraction Process
Precautions before starting the experiment:
- Reagent buffers A and C may precipitate under low-temperature conditions. It is recommended to heat at 65°C for 5 minutes and use after the precipitates have dissolved.
- WashBuffer 1 should have the specified amount of anhydrous ethanol added as indicated on the bottle label. Mark the label once ethanol has been added.
- Elution buffer is a 0.1x TE solutioncontaining minimal EDTA. If EDTA affects subsequent experiments, sterile deionized water is recommended as a substitute for the elution buffer.
- Sample Handling:
- Material Collection and Storage:
Freshly collected material, if not immediately used, should be placed in liquid nitrogen and ultimately stored at -80°C. Dried materials can be stored at room temperature.
- If possible, collect fresh material as it contains fewer polysaccharides and polyphenols.
- When collecting fungi from liquid culture, separate the liquid by centrifugation and collect the fungal bodies.
- Grind around 100 mg of fresh samples or no more than 20 mg of dry material using liquid nitrogen.
(Note: Different sample quantities may require optimization through preliminary experiments before use.)
- Dodati 550 μl of reagent buffer A and 10 μl of RNase A (10 mg/ml) to ensure there are no tissue clumps in the ground sample. Tissue clumps are difficult to lyse and can reduce DNA yield. Do not mix reagent buffer A and RNase Abefore use.
- Incubate at 65°C for 20-30 minuta, gently invert 2-3 times. This step is for cell lysis.
- Centrifuge the lysate for 5 minutes at 14,000 rpm (20,000×g).
(Note: Some plant materials may have a lot of sticky substances at this step, which can shear DNA in subsequent steps. Ideally, remove these substances by transferring the supernatant to a new centrifuge tube after centrifugation.)
- Carefully transfer the liquid obtained in the previous step to a new centrifuge tube.
(Note: Approximately 500 μl of liquid can be transferred; for some species, it may be less than 500 μl.)
- Add an equal volume of chloroform to the lysate and gently invert to mix.
(Note: For example, add 500 μl of chloroform if you have 500 μl of lysate. If the lysate volume is less than 500 μl, adjust the chloroform volume accordingly.)
- Centrifuge at 12,000 rpm for 10 minuta.
- Carefully transfer the supernatant to a new centrifuge tube (approximately 500 μl).
- Add an equal volume of reagent buffer C and an equal volume of anhydrous ethanol to the lysate, and mix.
(For example, if you add 450 μl of reagent buffer C, then add 450 μl of anhydrous ethanol. If the lysate volume is less than 450 μl, reduce the amount of reagent buffer C proportionally. Some precipitation will occur upon adding reagent buffer C, but it will not affect subsequent experiments.)
- Transfer the obtained liquid to a DNA purification column (kit), approximately 650-700 μl each time. Centrifuge at over 8,000 rpm for 1 minute, discard the collected waste, and reinsert the collection tube into the purification column for the next step.
- Repeat step 11, adding the remaining liquid to the DNA purification column (kit) and centrifuge at over 8,000 rpm for 1 minute. Discard the waste and the collection tube.
- Place the DNA purification column (kit) into a new collection tube, add 300 μl of Wash Buffer 1, centrifuge at over 8,000 rpm for 1 minute, discard the waste, and reinsert the DNA purification column (kit) into the tube for the next step.
(Note: Ensure that anhydrous ethanol has been added to Wash Buffer 1.)
- Dodati 500 μl of Rinse Buffer 1 to the DNA purification column (kit), centrifuge at 14,000 rpm (20,000×g) za 2 minuta, slightly prolong the centrifugation time for a drier membrane.
- Place the DNA purification column (kit) into a new centrifuge tube, open, and heat at 65°C for 2 minuta. This step may be prolonged to evaporate ethanol as much as possible to prevent residual ethanol from affecting downstream experiments.
- Drip 100 μl of elution bufferonto the membrane, centrifuge at 12,000 rpm for 2 minuta.
(Note: 1. Eluting DNA with 50 μl elution buffer can increase DNA concentration but decrease total DNA yield. 2. The eluate can be reapplied to the DNA purification column for a second elution, centrifuge at 12,000 rpm for 2 minutes to collect, which may improve DNA yield.)












Recenzije
Još nema recenzija.