Spectrometers and spectrophotometers are essential analytical instruments found in many scientific laboratories. But what exactly is the difference between a spectrometer and a spectrophotometer? These terms are often used interchangeably and can confuse. We will clearly explain how spectrometers and spectrophotometers are unique, their key features, and their applications.
What is a Spectrometer?
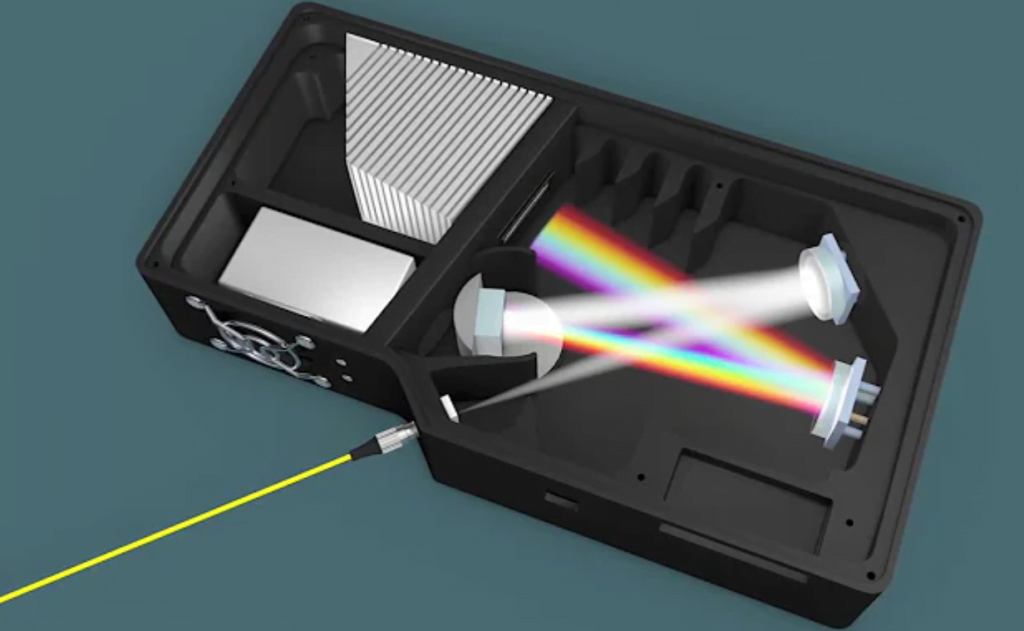
A spectrometer is an instrument that measures and analyzes the spectral composition of light across a specific portion of the electromagnetic spectrum. The key components of a spectrometer are:
- Light source: Generates light to be applied to the sample. Common sources are tungsten lamps, LEDs, lasers, itd. depending on the wavelength needed.
- Wavelength selector: Contains a prism or diffraction grating that separates polychromatic light into different wavelengths or colors.
- Sample holder: Houses the sample material to be analyzed.
- Detector: Measures the intensity of light at different wavelengths after interaction with the sample.
- Display: Shows the spectral data, often using a graph of intensity versus wavelength.
By separating light into component wavelengths and measuring intensity, spectrometers characterize how a sample absorbs, emits, or scatters light. This reveals properties and chemical composition.
What is a Spectrophotometer?
A spectrophotometer is an instrument that quantitatively measures the transmission or absorption of light passing through a sample. It contains a spectrometer for wavelength selection and intensity measurements. The key additional component is a photometer which measures light intensity.
In spectrophotometers, the spectrometer splits light into wavelengths that then pass through the sample. The photometer detects how much light is absorbed. The microprocessor converts the signals into absorbance or transmission values.
Spectrophotometers allow both qualitative and quantitative analysis of samples based on their interaction with light. They are commonly used to determine concentration, identify analytes, and study kinetic reactions.
What’s the Difference?
Spectrometers and spectrophotometers are closely related, but have some key differences:
- Purpose: Spectrometers characterize light composition; spectrophotometers quantify light absorption.
- Measurement: Spectrometers measure emission/intensity; spectrophotometers measure absorbance/transmission.
- Components: Spectrometers have wavelength selectors and detectors; spectrophotometers add a photometer.
- Data: Spectrometers show intensity spectra; spectrophotometers give absorption values.
- Uses: Spectrometers identify molecules; spectrophotometers determine concentration.
So while a spectrophotometer contains a spectrometer, it also has a photometer and produces quantitative absorbance data used to analyze samples.
How Does a Spectrometer Work?
Spectrometers work by dispersing light into component wavelengths and measuring the intensity at each wavelength. The operating principle includes:
- The light source emits a broad spectrum of light.
- The wavelength selector (prism or grating) splits the light into separate wavelengths.
- The sample interacts with the light via absorption, emission, or scattering.
- The detector measures the intensity of light at each wavelength.
- The microprocessor generates a spectrum with intensity plotted versus wavelength.
Analysis of emission or absorption peaks in the spectrum reveals information about the sample composition and properties.
How Does a Spectrophotometer Work?
Spectrophotometers build on spectrometer components to quantify light absorption by samples:
- The spectrometer splits light across wavelengths.
- The monochromatic light passes through the sample in a cuvette.
- The photometer detects how much light is transmitted through or absorbed by the sample.
- Transmittance (%) or absorbance values are displayed or printed.
- Wavelengths are scanned automatically to produce an absorption spectrum.
By precisely measuring light absorbance, the concentration, kinetics, and properties of samples can be determined.
What is Spectrometry?
Spectrometry refers to the quantitative measurement and analysis of spectra produced by spectrometers or spectrophotometers. The suffix “-metry” denotes the act of taking a measurement.
Applications of spectrometry include:
- Identifying molecules based on emission/absorption spectra
- Determining unknown concentrations using calibration curves
- Monitoring reaction kinetics by following spectral changes over time
- Evaluating sample properties like color, fluorescence, itd.
Spectrometry produces the actual numerical spectral data used for analysis and interpretation.
What is Spectroscopy?
Spectroscopy refers to the study of how matter interacts with electromagnetic radiation. It is primarily a qualitative approach focused on understanding absorption and emission characteristics.
Types of spectroscopy include:
- Atomic absorption/emission spectroscopy
- Vibrational spectroscopy (infrared, Raman)
- Nuclear magnetic resonance (NMR) spectroscopy
- Electron spectroscopy
- Fluorescence spectroscopy
Spectroscopy establishes relationships between spectral behavior and sample properties, composition, and structure. Međutim, spectrometers and spectrophotometers are needed to acquire experimental spectroscopic data.
What Wavelength Ranges are Measured?
Spectrometers and spectrophotometers are designed to operate over certain wavelength regions:
- Ultraviolet (UV):200-400 nm
- Visible:400-700 nm
- Near-infrared (NIR):700-2500 nm
- Mid-IR:2500-25000 nm
- Far-IR:25-1000 μm
Specific light sources, wavelength selectors, and detectors are chosen based on the desired spectral range. UV-vis, IR, and fluorescence spectrophotometers are common configurations.
What are the Key Components?
Spectrometers and spectrophotometers share the same core components:
Light Sources
- Tungsten-halogen, deuterium, and xenon arc lamps for UV-vis range
- Infrared emitting sources like globals for the IR range
- Lasers for Raman spectroscopy
Wavelength Selectors
- Prisms, diffraction grating monochromators, or filters
Sample Holders
- Cuvettes, vials, holders, or ports for solid, liquid, and gas samples
Detectors
- Photodiodes, CCDs, photomultiplier tubes (PMTs)
Display and Software
- Screen, printouts, and computer interfaces to acquire and analyze data
What are the Key Differences in Components?
The main distinguishing component between spectrometers and spectrophotometers is the photometer. Spectrophotometers contain a dedicated photometer to accurately quantify light intensity after interacting with the sample. This allows absorbance or transmittance values to be determined.
Spectrometers specialized for imaging may utilize multi-element CCD detectors or camera systems rather than single-point photometers. They produce spectral imaging data over a surface.
What Types of Spectrophotometers Are There?
Some common types of spectrophotometers include:
- UV-vis spectrophotometer: Measures light absorption in UV and visible ranges (200-800 nm). Used for quantification of many inorganic and organic compounds.
- Infrared spectrophotometer: Measures infrared light absorption, allowing identification of chemical bonds and functional groups.
- Atomic absorption spectrophotometer (AAS): Uses absorption of light by vaporized analyte atoms to determine concentrations of metals and metalloids.
- Fluorescence spectrophotometer: Measures intensity of fluorescent light emitted from samples after excitation. Allows highly sensitive analysis of samples with native or induced fluorescence.
- Colorimeter: Simple spectrophotometers used to measure light absorption for colorimetric assays and tests.
What are Spectrophotometers Used For?
Spectrophotometers allow both quantitative and qualitative analysis in a wide range of fields:
- Determining unknown concentrations using Beer’s law
- Monitoring kinetics of reactions over time
- Identification of compounds based on absorption spectra
- Quality control and production monitoring
- Analysis of pharmaceuticals, foods, chemicals
- Protein and DNA quantification
- Medical diagnoses and clinical assays
- Color measurement
From biochemistry labs to manufacturing plants, spectrophotometers provide fast and reliable analytical capabilities.
What are Spectrometers Used For?
Spectrometers also have diverse applications across many fields:
- Identification of molecules based on emission and absorption spectra
- Analysis of X-rays, gamma rays, and charged particles
- Determining elemental composition and isotope ratios
- Astronomical observation and space exploration
- Measuring spectral radiance of light sources
- Monitoring air and water quality
- Remote sensing and hyperspectral imaging
Spectrometers give fundamental information about sample composition, structure, energetics, and physical processes.
Conclusion
Spectrophotometers and spectrometers are indispensable tools for gathering qualitative and quantitative spectroscopic data across diverse fields. While closely related, understanding the key distinctions allows the selection of the most appropriate instrument for the intended application. Properly utilizing these technologies provides the spectroscopic insight needed to drive discoveries, innovations, and breakthroughs