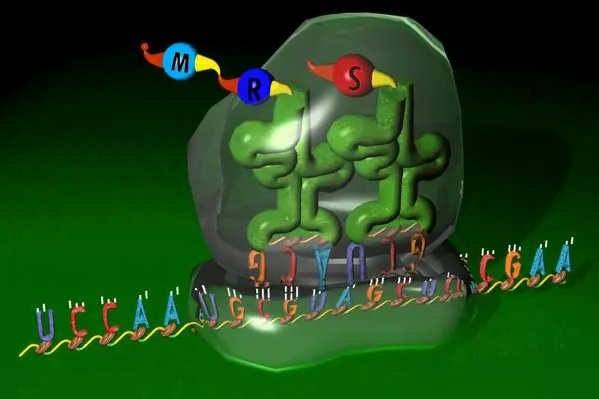
Ribosomal RNA, also known as rRNA, constitutes over 50% of the total RNA content in a typical cell and forms a key component of ribosomes. It exists in three primary types – 5S, 5.8S, 16S, and 28S rRNA in eukaryotes or 5S, 16S, and 23S rRNA in prokaryotes. Together with ribosomal and other associated proteins, these rRNA molecules fold up and assemble into the characteristic structures of the small and large ribosomal subunits.
In addition to providing much of the physical scaffolding for ribosome architecture, distinct functional regions within the diverse rRNA molecules directly facilitate core processes of translation. The 16S and 23S rRNAs situate in the small 30S subunit where they recognize initiation codons on mRNA and recruit other translation factors. Their conserved sequences form molecular pockets that precisely pair messenger nucleotides for the initial decoding of genetic messages.
Meanwhile, catalytic centers within the 23S and 28S rRNAs in the large 60S subunit drive the formation of peptide bonds between incoming amino acids. Through a set of coordinated nucleophilic attacks and proton transfers, these functional rRNA domains join the carboxyl group of one amino acid to the amino group of another, polymerizing new proteins. Other rRNA regions help stabilize transition state intermediates and aminoacylated transfer RNAs (tRNAs) during elongation.
What role does rRNA play in ribosomes?
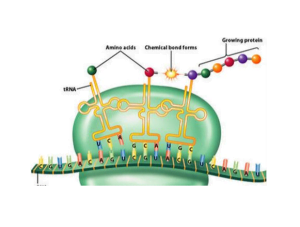
Ribosomal RNA, also called rRNA, makes up around 60% of the total ribosome. It serves both a structural and catalytic function. The various rRNA molecules provide the physical scaffolding that holds the two ribosome subunits together.
Inoltre, specific regions of rRNA are enzymatically active and directly facilitate the reactions involved in linking amino acids together during protein assembly. Without rRNA, the ribosome would not have the appropriate 3D architecture or biochemical abilities to perform its duties of protein production.
How does translation initiation occur on ribosomes?
The first major step in protein synthesis is translation initiation. This involves the small ribosomal subunit binding to the start codon of messenger RNA (mRNA) with the help of initiation factors.
The 16S rRNA within the small subunit recognizes and pairs with the Shine-Dalgarno sequence on mRNA, allowing it to slide into position. This 16S rRNA acts as a molecular barcode reader to accurately locate the initiation site.
What happens next in the translation process?
Once translation initiation is complete, elongation can begin. The large ribosomal subunit joins the small one with the help of elongation factors.
Transfer RNA (tRNA) molecules carrying activated amino acids are matched to the mRNA codons. The 23S rRNA in the large subunit catalyzes peptide bond formation between the incoming amino acid and the growing polypeptide chain.
This process of aminoacylation, selection, accommodation and peptide bond formation repeats itself along the mRNA template until a stop codon is reached and translation termination occurs.
How are different rRNA types characterized?
All ribosomes contain distinct rRNA molecule types that adopt characteristic higher-order structures and precisely organize within subunits to perform non-redundant functions. In most bacteria, these consist of 5S, 16S and 23S rRNAs while eukaryotes also have 5.8S and 28S varieties. Biochemical analyses have revealed unique properties of each:
5S rRNA – UN 120 nucleotide RNA that binds ribosomal proteins and folds into a compact 5-helix bundle that packs into the central protuberance region of the 50S subunit. Provides a scaffold and helps coordinate intersubunit movements.
16S rRNA – At ~1500 nucleotides, it forms the structural core of the 30S subunit. Contains four distinct domains that fold into Secondary structure elements and interdomain bridges critical for mRNA and tRNA interactions.
23S rRNA – The largest rRNA at ~2900 nucleotides. Within the 50S subunit, it adopts a characteristic overall L shape with three domains containing many highly conserved nucleotide sequences. These nucleic acid pockets adopt conformations suited to catalyze peptide bond formation.
5.8S and 5S rRNA – Reside in the 60S subunit. 5.8S rRNA assumes a hairpin-helix-hairpin structure and aids assembly. The 5S rRNA, while smaller, is similarly structured.
28S rRNA – Eukaryotic equivalent of 23S rRNA that contributes to the active site. Additional expansion houses additional peptide transfer machinery and regulatory elements.
Combining structural, mutational and crosslinking studies has revealed precise locations of each rRNA type within subunits and illustrated how their distinct topologies cooperate during decoding, proofreading and protein production.
How is 16S rRNA used in microbiology?
16S rRNA gene sequencing revolutionized microbiology by enabling cultivation-independent microbial identification and classification. Regions within the 16S rRNA molecule called the hypervariable regions (V1-V9) show variability in nucleotide sequence between organisms but are highly conserved within species. This makes the V regions ideal molecular markers.
By PCR amplifying 16S rDNA from an environmental sample and determining the sequence of say the V3-V5 regions, the obtained signature can be run through BLAST against reference databases of characterized microbial taxa. The database hit with the closest match indicates the phylogenetic identity of the formerly uncultivated microbe.
This powerful technique helped define the tree of life by grouping newly encountered microbes based on rRNA gene evolutionary relatedness. It allowed rapid detection of pathogens from clinical and food samples without culturing. Scientists used it to explore previously hidden diversity in exotic environments like acidic mine drainage or hydrothermal vents.
More recently, high-throughput 16S rRNA amplicon sequencing revolutionized microbiome research. It enables profiling entire microbial communities directly from microbial DNA in samples without bias from selective culturing. Output from next-gen sequencing platforms is processed using bioinformatics pipelines to assign sequences taxonomically and compare microbial assemblages between environments, host niches or treatment groups.
Limitations include the inability to resolve some species and primer biases influencing coverage. Other marker genes are now explored too, but 16S rDNA analysis remains the gold standard due to database depth. It continues stimulating countless discoveries by shining light into the dark realm of microbial diversity on Earth. Applications range from diagnosis to bioprospecting and understanding the roles of microbes in ecosystems, health, and disease.
Does rRNA provide insight into the origins of life?
Some scientists speculate rRNA may have played a pivotal role in the very first life forms on Earth before the emergence of DNA and complex cells. As a self-replicating and functionally active molecule forming the basis of protein synthesis, rRNA or its ancestors could represent a scenario for how primitive life bootstrapped its way to greater complexity over billions of years of evolution.
Ongoing research aims to uncover clues by learning more about rRNA structure-function relationships across diverse organisms living today and how those evolved in step with metabolic and cellular innovations from the ancient past to the present.
What are Advanced techniques for studying rRNA?
Given rRNA’s complexity, diverse methods are employed to illuminate its myriad structure-function relationships at varied scales:
- Next-gen sequencing determines rRNA gene sequences across domains, aiding taxonomy and characterization of variants between species.
- Cryo-electron microscopy and X-ray crystallography yield near-atomic resolution structures of entire ribosomes or domains, revealing rRNA-rRNA and rRNA-protein interactions.
- Chemical probing maps nucleotide involvement in folding, conformational changes and interactions with ligands through sites of reactivity to modifying agents.
- Crosslinking coupled with mass spectrometry pinpoints rRNA regions contacting specific proteins or other biomolecules within assembled particles or reaction intermediates.
- Single-molecule FRET monitors the folding of individual rRNA molecules and detects conformational shifts during biochemical processes like translocation.
Integrating multi-scale insights from advanced technologies is unraveling rRNA’s intricate structure-function relationships essential for translation and cellular homeostasis with unprecedented depth. Its fundamental importance inspires ongoing innovation.
In summary, while DNA is most famous as the core genetic material of life, it is the unsung hero – rRNA – that ensures the critical step of transforming DNA code into the functional molecules that allow cells and organisms to survive through its essential role in protein production via ribosomes. rRNA is truly the indispensable engine of life itself.