A spectrophotometer is an important analytical instrument found in many chemistry and biology labs. This versatile tool measures the interaction between light and matter, providing valuable quantitative information about samples. But what exactly does a spectrophotometer do? How does it work? And what can you use it for? This guide will explain the key principles behind spectrophotometry and how to properly use these devices.
What is a Spectrophotometer?
A spectrophotometer is an optical instrument designed to measure the transmission or absorption of light passing through a sample. It can determine how much light is absorbed by a chemical solution at different wavelength ranges.
The key components of a basic spectrophotometer are:
- 光源: Generates the light beam applied to the sample. Common sources include tungsten-halogen, deuterium, xenon lamps, and LEDs.
- Monochromator: Contains a prism or diffraction grating that separates polychromatic light into different wavelengths.
- Wavelength selector: Filters the light to transmit only a narrow band of wavelengths.
- Sample compartment: Houses the cuvette containing the sample liquid.
- 検出器: Measures the intensity of light passing through the sample. Photodiodes, photomultiplier tubes, and photoresistors are typical detectors.
- Display: Shows the absorption data, often in transmittance percentage or absorbance units.
Spectrophotometers allow quantitative analysis of samples based on their interaction with light. They can identify substances, determine concentrations, and evaluate sample properties.
What Does a Spectrophotometer Measure?
Spectrophotometers provide two main measurements:
Transmittance
This is the amount of light that passes through a sample, expressed as a percentage. If a sample transmits 80% of the light, it has a transmittance of 80%. The transmittance depends on the wavelength.
Higher transmittance means more light passes through. Transparent solutions tend to have high transmittance. Opaque-absorbing samples transmit less light and have lower transmittance.
Absorbance
The absorbance measures how much light is absorbed by the sample. It is based on the transmittance using the equation:
A = -log T
Where A is absorbance and T is transmittance. Absorbance has no units. Liquids that absorb light strongly have high absorbance values. Weak absorbing samples give low absorbance readings.
By measuring how transmittance and absorbance vary with different wavelengths, the spectrophotometer produces an absorption spectrum for the sample. This spectrum acts like a molecular fingerprint to identify analytes.
How Does a Spectrophotometer Work?
Spectrophotometers operate on the principle that different substances absorb and transmit light differently at various wavelengths. Here is an overview of the measurement process:
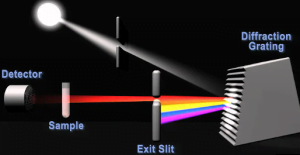
1. Generate Light
The light source emits a broad spectrum of light. Common optical sources include incandescent lamps for visible and near-infrared light or deuterium arc lamps for ultraviolet wavelengths.
2. Select Wavelength
The monochromator selects a narrow band of wavelengths to pass through. Common monochromators have prisms or diffraction gratings to disperse the light.
3. Pass Light Through Sample
The beam of monochromatic light is transmitted through the sample contained in a clear quartz cuvette. Some energy is absorbed by the sample.
4. Detect Transmitted Light
A photodetector measures how much light passes through the sample. Photodiodes and photomultiplier tubes are typical detectors.
5. Measure Absorbance
The ratio of transmitted to incident light intensity gives the transmittance percentage. This is converted into an absorbance value and displayed.
6. Scan Wavelengths
Steps 2-5 are repeated automatically across a range of wavelengths to produce the absorption spectrum.
By measuring absorbance at different wavelengths, the composition, 集中, and properties of the sample can be determined.
What is Spectrophotometry Used For?
Spectrophotometry has become an essential analytical technique used in many fields:
- Chemistry: Identifying compounds, quantifying analytes, evaluating reactions
- Biochemistry: タンパク質, enzyme, and DNA assays and analysis
- Microbiology: Cell density measurements, growth studies
- Industry: Quality control, manufacturing process monitoring
- Medicine: Clinical chemistry, diagnostic tests
Some common applications of spectrophotometers include:
- Measuring solution concentration using Beer’s law
- Evaluating the purity of pharmaceuticals
- Analyzing food and beverages
- Monitoring industrial processes
- Determining chemical kinetics
- Quantifying DNA and proteins
- Identifying pathogens and toxins
- Clinical diagnosis tests
Spectrophotometry provides fast, affordable, and sensitive quantitative analysis of samples across diverse fields.
How to Use a Spectrophotometer
Using a spectrophotometer properly is vital for accurate results. Here are some key guidelines for using spectrophotometers:
Prepare Samples
- Make sure samples are completely dissolved and homogeneous solutions. Filter or centrifuge to remove any particulates.
- Use quartz cuvettes, matched to the specified light path length. Clean thoroughly and handle carefully.
- Prepare a blank containing just the solvent for reference. Zero or baseline correct using the blank.
Select Wavelengths
- Choose wavelengths where the sample absorbs light for detection. Scan across a range to identify maximum absorption peaks.
- Avoid regions where the sample does not absorb for measurements. This wastes light energy.
Follow Procedures
- Read and follow all instrument instructions. Spectrophotometers have different makes, models, software, and accessories.
- Develop and validate detailed standard operating procedures for repeatable results.
Analyze Data
- Ensure proper calibration using reference standards. Create calibration curves if needed.
- Average multiple measurements for accuracy. Use statistical analysis to determine precision.
- Correct for any background interference from solvents or cuvettes.
With training and experience, researchers can fully leverage spectrophotometers for reliable quantitative sample analysis.
Types of Spectrophotometers
There are several different types of spectrophotometers used in laboratories:
UV-Visible Spectrophotometers
- Measure light absorption in ultraviolet and visible ranges (~200-800 nm)
- Use tungsten-halogen lamps, deuterium lamps, or LEDs as light sources
- Analyze transition metal complexes, biological samples, and visible dyes
Infrared Spectrophotometers
- Operate in infrared wavelength region (~750 nm – 300 μm)
- Utilize infrared emitting sources like the Nernst glower
- Identify functional groups and analyze the structure of compounds
Atomic Absorption Spectrophotometers
- Measure absorption of light by vaporized elements
- Require a flame or graphite furnace atomizer
- Determine metals and metalloids in samples
Fluorescence Spectrophotometers
- Measure the intensity of emitted fluorescent light from samples
- Use a xenon lamp or LED to provide excitation light
- Analyze samples with natural or induced fluorescence
Raman Spectrophotometers
- Detect Raman scattered light from laser excitation
- Provide vibrational and rotational modes of molecules
- Identify samples and quantify analytes
Selecting the right spectrophotometer depends on your wavelength region of interest and the types of samples to be measured.
結論
Spectrophotometers have become indispensable analytical instruments across many fields. By quantifying light interaction with samples, they provide invaluable composition and property data rapidly and sensitively.
Understanding what spectrophotometers measure, how they work, and proper usage techniques allow researchers to harness their full potential. With ongoing advances in detector technology, light sources, and data analysis, spectrophotometers will continue driving scientific discovery into the future.
