- Components of the reagent kit
| Specifications | 50T | 100T |
| Cat. No. | SN0305AD | SN0306AD |
| RNA Extraction Columns (set) | 50 (set) | 100 (set) |
| DNA Clean-Up Columns (set) | 50 (set) | 100 (set) |
| RNA Extraction Buffer I | 30 ml | 2×30 ml |
| Inhibitor Removal Buffer | 30 ml | 2×30 ml |
| Wash Buffer 1 | 15 ml | 2×15 ml |
| Elution Buffer | 20 ml | 20 ml |
| Instruction Manual | 1 | 1 |
- Storage
This reagent kit can be stored at room temperature (15-25℃) in a dry environment and is stable for 12 months.
- Instructions for Using the Reagent Kit
3.1 This kit is intended for molecular biology research purposes and should not be used for disease diagnosis or treatment.
3.2 Some components in the kit contain irritants; it is advisable to take necessary precautions (such as wearing protective clothing and goggles).
3.3 The use of this kit requires additional equipment such as a high-speed centrifuge, water bath (metal bath), vortex mixer, anhydrous ethanol, liquid nitrogen, chloroform, sterile deionized water, and EP tubes.
- Introduction to the Reagent Kit
This RNA purification kit provides a fast and efficient method for purifying polysaccharide and polyphenol plant total RNA, suitable for most polysaccharide and polyphenol-rich plant tissues. In general, plant tissues rich in polysaccharides and polyphenols contain a high level of secondary phenolic metabolites and polysaccharides, significantly affecting RNA extraction efficiency. This kit can effectively remove polysaccharides and polyphenols, as well as efficiently eliminate DNA contamination from samples. If the experiment is sensitive to DNA, it is recommended to use primers spanning introns for downstream experiments.
The RNA Fast Purification Kit can extract total RNA from plant tissues (including nuclear RNA and cytoplasmic RNA) within 1 hour. The extracted RNA can be directly used for RT-PCR, Northern blotting, etc. The entire purification process does not require toxic reagents such as phenol-chloroform, making this RNA purification kit suitable for various other sample types.
- Experimental Principles and Procedures
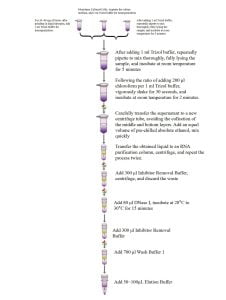
- Extraction Process
Precautions before starting the experiment:
A. Before using Wash Buffer 1, add the specified amount of absolute ethanol according to the label on the reagent bottle, and check the box on the label to indicate that absolute ethanol has been added.
B. The Elution Buffer is a 0.1x TE solution containing minimal EDTA. If EDTA affects subsequent experiments, it is recommended to replace the Elution Buffer with sterile deionized water.
- Sample Processing:
A. Material Collection and Storage:
If fresh materials cannot be used immediately, place them in liquid nitrogen and store them at -80°C.
B. Whenever possible, collect fresh materials, as they contain fewer polysaccharides and polyphenols.
2. Grind approximately 100 mg of fresh samples or not more than 20 mg of dry material using liquid nitrogen.
3. Add 600μl RNA Extraction Buffer I, ensuring there are no blocky tissues in the ground sample. Blocky tissues are difficult to lyse and can reduce RNA yield.
4. Vortex for 30 seconds.
5. Centrifuge the lysate for 5 minutes at 12,000 rpm.
(Note: Polysaccharide plant materials may produce a lot of sticky substances at this step, which can shear RNA in subsequent steps. Therefore, remove these substances during this step.)
- Transfer the supernatant obtained in the previous step to a DNA Clear Purification Column, centrifuge at 12,000 rpm for 30 seconds, and collect the filtrate (Note: RNA is present in the filtrate).
- Add 250μl of absolute ethanol, mix by pipetting. If there is a small amount of precipitation, it does not affect subsequent experiments. Add the liquid to the RNA purification column, centrifuge at 12,000 rpm for 30 seconds, and discard the flow-through.
- Add 700μl Inhibitor Removal Buffer to the RNA purification column, centrifuge at 12,000 rpm for 30 seconds, and discard the flow-through.
- Add 700μl Wash Buffer 1 to the RNA purification column, centrifuge at 12,000 rpm for 30 seconds, and discard the flow-through.
- Add 500μl Wash Buffer 1 to the RNA purification column, centrifuge at 12,000 rpm for 3 minutes, and discard the flow-through.
- Place the RNA purification column into a new 1.5ml centrifuge tube, air-dry the membrane at room temperature for 2 minutes.
(Note: Confirm the addition of ethanol to Wash Buffer 1. The presence of ethanol significantly affects subsequent experiments. Therefore, membrane drying is crucial. After centrifugation, ensure the absence of ethanol before elution. Discard the waste and the collection tube. After using Wash Buffer 1, the membrane on the RNA purification column should only have a slight color. Carefully remove the RNA purification column after centrifugation, ensuring it does not touch the collection tube to avoid ethanol contamination.)
- Drip 50-100μl Elution Buffer onto the membrane, centrifuge at 12,000 rpm for 1 minute, and collect the RNA solution.
(Note: Eluting RNA with 50μl Elution Buffer can increase RNA concentration but decrease total RNA yield.)








Reviews
There are no reviews yet.