Product Overview
| Attribute | Details |
|---|---|
| Cat | CA1030 |
| Size | 20T/50T/100T |
| Storage | Store at 2-8°C protected from light, do not freeze |
| Product Content: | 20T | 50T | 100T |
| 4 x (Binding Buffer 4x) | 4 ml | 10 ml | 20 ml |
| 7-AAD Viability Staining Solution | 0.2 ml | 0.5 ml | 1.0 ml |
| rh Annexin V/PE | 0.1 ml | 0.25 ml | 0.5 ml |
Product introduction:
- Early changes in apoptosis occur on the cell membrane surface.
- One of these changes is the transfer of phosphatidylserine (PS) from the inside to the outside of the cell membrane, exposing PS on the outer surface.
- PS is a negatively charged phospholipid primarily found on the inner surface of the cell membrane.
- During apoptosis, the asymmetry of phospholipid distribution is disrupted, exposing PS externally.
- Annexin V binds easily to PS and has a high affinity for it, making it a sensitive probe for detecting PS on the cell membrane surface.
- PS transfer to the outer membrane is not exclusive to apoptosis but can also occur in cell necrosis.
- The key difference is that the cell membrane remains intact in the early stages of apoptosis, whereas it is compromised in early cell necrosis.
- Thus, the double staining method of Annexin V and 7-AAD can be utilized to detect early cell apoptosis via flow cytometry.
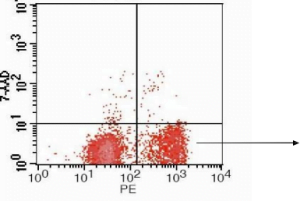
Flow cytometric analysis of Jurkat cells using Annexin V-PE/7AAD after inducing apoptosis with cisplatin
Method of operation: (for reference only)
- Preparation of cell samples:
- For adherent cells: carefully collect the cell culture medium into a centrifuge tube for later Digest the cells with trypsin without EDTA. When the cells can be gently pipetted down with a pipette or pipette tip, add the previously collected cell culture solution, pipette down all the adherent cells, and gently blow off the cells. Collect again into the centrifuge tube. Centrifuge at about 1000 rpm for 5 minutes to pellet the cells. For specific cells, if the cells cannot be completely centrifuged to the bottom of the centrifuge tube, you can appropriately extend the centrifuge time or slightly increase the centrifugal force. Carefully aspirate the supernatant. About 50μl of culture fluid can remain to avoid aspirating the cells. Add about 1ml of 4℃ pre-cooled PBS, resuspend the cells, centrifuge again to pellet the cells, and carefully aspirate the supernatant;
- For suspension cells: Centrifuge at about 1000 rpm for 5 minutes to pellet the cells. For specific cells, if the cells cannot be completely centrifuged to the bottom of the centrifuge tube, you can appropriately extend the centrifuge time or slightly increase the centrifugal force. Carefully aspirate the About 50μl of culture fluid that can remain to avoid aspirating the cells. Add about 1ml of 4℃ pre-cooled PBS, resuspend the cells, centrifuge again to pellet the cells, and carefully aspirate the supernatant;
- Dilute the binding buffer 1:3with deionized water (4ml 4x binding buffer + 12ml deionized water);
- Resuspend the cells with 1x binding buffer and adjust the concentration to1-5×106/ml;
- Take 100µl of cell suspension into a 5ml flow tube, add 5µl Annexin V/PE to mix well, and incubate for 5 minutes at room temperature in the dark;
- Add 10µl of 20ug/ml 7AAD and 400µl of PBS to perform flow detection
Experimental design:
Untransfected cells
- Blank tube: Negative control cells, without Annexin V/PE, 7AAD, used to adjust voltage.
- Single staining tube: positive control cells, only with Annexin V/PE or only 7AAD for adjustment and compensation.
- Detection tube: treated cells, add Annexin V/PE, 7AAD. After adjusting the voltage compensation with the blank tube and the single dye tube, the required flow data can be obtained.
Transfection with GFP
- Untransfected blank tube: untransfected cells, without Annexin V/PE, 7AAD, were used to adjust the voltage.
- Untransfected single-stained tube: For untransfected cells with obvious apoptosis, only add Annexin V/PE or only 7AAD for adjustment and compensation.
- Transfection of GFP blank tube: Transfect GFP control cells without Annexin V/PE, 7AAD for adjustment and compensation.
- Detection tube: treated cells, plus Annexin V/PE, 7AAD. After adjusting the voltage compensation, obtain the required streaming data.
Precautions:
- AnnexinV is compatible with phosphatidylserine (PS), and PS has no difference between different species. In normal cells, PS is only distributed on the inner side of the lipid bilayer of the cell membrane. In the early stage of apoptosis, PS turns from the inner side of the lipid membrane to the outer side.
- Digest with low-concentration trypsin, gently blow the adherent cells 2 to 3 times, and centrifuge at 4°C 1000 rpm for 5 minutes, if handled properly, the damage caused by trypsin can be controlled within 5%. If there is a control group, the experimental results Will not cause significant
- Add PI first. Not only is it difficult to judge whether the staining is uniform and sufficient for each group, but PI itself is also toxic to cells and will have a greater impact on the experimental results than pancreatin. This is not recommended.
- AnnexinV is a Ca-dependent protein, so EDTA cannot be added to prevent EDTA from chelating Ca ions and thus affecting Annexin V, thereby affecting the
- When using flow cytometry to detect apoptosis, 7AAD is greatly affected by time. Because 7AAD is labeled, it will increase cytotoxicity. As time goes on, it will increase the staining of 7AAD, especially when detecting early apoptosis. In addition to increasing the gap between the cell populations on the flow cytometer, the error will increase significantly. Generally, 7AAD is added and the machine is immediately on the machine, and then the test is completed within a Both methods are ok, but the error caused by following our operating steps will be smaller.
Related literature:
[1] Ruifeng Zhao, Jing Jin, Xinyu Sun, et al. The establishment of a clonally derived chicken embryonic fibroblast cell line (CSC) with high transfection efficiency and ability as a feeder cell. Journal of Cellular Biochemistry. August 2018. (IF 2.959)


Reviews
There are no reviews yet.