Total antioxidant capacity (T-AOC) Assay Kit
Note: Take two or three different samples for prediction before test.
Operation Equipment: Spectrophotometer
Cat No: BC1310
Size:50T/48S
Components:
Extract solution: Liquid 50 mL×1. Storage at 4℃, precool before use.
Reagent I: Liquid 35 mL×1. Storage at 4℃.
Reagent II: Liquid 20 mL×1. Storage at 4℃ in shadow.
Reagent III: Liquid 5 mL×1. Storage at 4℃ in shadow.
Standard: Powder×1, 10 mg of FeSO4·7H2O. Working solution: add 0.9 mL of distilled water and 20μL of concentrated sulfuric acid (H2SO4) to forms 40 µmol/mL FeSO4 standard solution. Solution mixture (prepare when the solution will be used): Reagent I : Reagent II: Reagent III= 7:1:1, incubate at 37℃ before use.
Product Description:
This kit is used to detect the total antioxidant levels of antioxidants and antioxidant enzymes in the samples. It is mainly used in the study of biological, medical and pharmaceutical studies to detect the total antioxidant capacity of antioxidant solutions.
In acid environment, Fe3+ -TPTZ are reduced to blue Fe2+ -TPTZ. The color reaction reflects the total antioxidant capacity.
Reagents and Equipment Required but Not Provided:
Spectrophotometer, constant temperature water bath, low temperature centrifuge, 1 mL glass cuvette and distilled water.
Procedure:
I. Sample preparation:
1. Serum, plasma, saliva, or urine samples
Plasma (anticoagulation with heparin or sodium citrate, avoid using EDTA), centrifuge at 5000 rpm/min for 10 min, take supernatant for test. Take serum, saliva or urine samples for direct determination. Also, you can store at -80℃ and detect within 30 days.
2. Cells or tissue sample
Take 1-2 million cells or 0.1 g of tissue, add 1.0 mL of Extract solution. Use homogenate or ultrasound to fully break up cells and release antioxidant, centrifuge at 10000 r/min and 4℃ for 5 min, take supernatant for test. Measure the concentration of protein if needed.
II. Determination procedure:
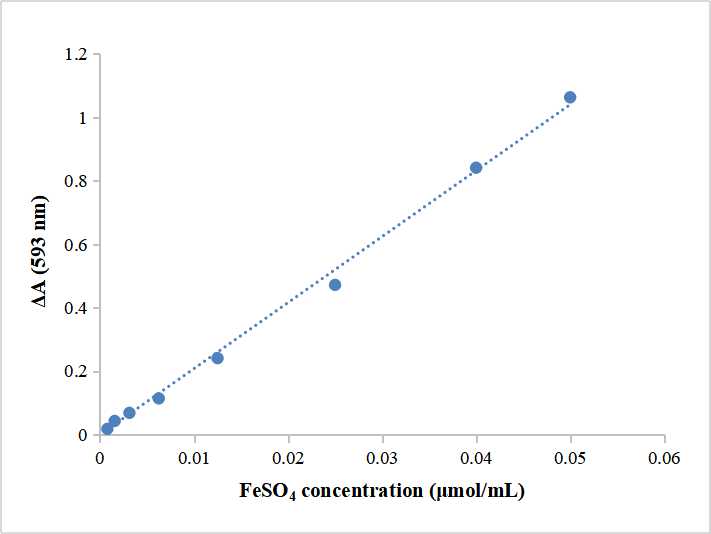
- Dilute 40 μmol/mL FeSO4 standard solution to 0.1, 0.05, 0.025, 0.0125, 0.00625, 0.003125 μmol/mL, take 500 μL of standard solution (distilled water for blank control), add to 500 μL of Reagent II. Mix thoroughly for 10 min, detect the absorbance in 593 nm, calculate ΔA=AS-AB. (AS: standard solution tube, AB: blank control tube.) The final concentration of Fe2+ is 0.05、0.025、0.0125、0.00625、
0.003125、0.00156 μmol/mL.
- Preheat the spectrophotometer 30 min, adjust wavelength to 593 nm and set zero with distilled
- Add reagents with the following list:
| Reagent Name | Blank tube (AB) | Test tube (AT) |
| Solution mixture (μL) | 900 | 900 |
| Sample (μL) | 30 | |
| Double distilled water (μL) | 120 | 90 |
| Mix thoroughly and react for 10 min, set zero with distilled water, detect the absorbance in 593 nm. Calculate ΔA’=AT – AB. (Note: The blank tube just needs to be tested once or twice in every experiment) | ||
III. Calculation:
- Create standard curve
Take the Fe2+ final concentration as X-axis, △A as Y-axis, create standard curve, get linear regression equation y=kx+ b, take ΔA’ into the equation to get x (μmol/mL).
- Unit definition: the sample antioxidant capacity is indicated by the standard liquid ion concentration required for the same absorbance change(ΔA).
A. Protein concentration:
Total antioxidant capacity (μmol/mg prot) = x × Vrv÷ (Vs× Cpr) = 34× x ÷ Cpr
B. Sample weight
Total antioxidant capacity (μmol/g weight) = x × Vrv÷ (Vs ÷ Vsv ×W) =34× x÷ W
C. Cell amount
Total antioxidant capacity (μmol/104cell) = x × Vrv÷ (Vs ×Vsv÷n) = 34× x÷ n
D. Solution volume
Total antioxidant capacity (μmol/mL) =x× Vrv÷ Vs =34×x
Vrv: total reaction volume, 1.02 mL; Vs: sample volume, 0.03 mL;
Vsv: extraction volume, 1 mL; W: sample weight, g;
Cpr: sample protein concentration, mg/mL;
n: cell amount, unit based on 104 (ten thousand).
Note:
- Reagent II is irritated to human body, please wear lab clothes and latex
- The samples should not be appeared blue under acidic condition, or it will interference sample result of the
- Detergent such as Tween, Triton, NP-40 and reductants such as DTT, mercapto ethanol should not be added in the
- If the absorbance value determined by the sample is beyond the standard curve range, the sample should be diluted or concentrated properly before
- The kit should be store at2-8℃.
Examples:
- Add 0.1g shamrock to 1mL extract solution and grind thoroughly on ice, take supernatant, follow the determination procedure to operate, with 96-well flat-bottom plates to calculate: ΔA=A(T)-A(B)=0.909-0.148=0.761, standard curve: y=21.056x-0.0087, calculate x=0.037, according to mass of sample to calculate Total antioxidant capacity (μmol/g mass) =34×x÷W=34×0.037÷0.1=12.85 μmol/g mass.
References:
[1] Pellegrini N, Serafini M, Salvatore S, et al. Total antioxidant capacity of spices, dried fruits, nuts,
pulses, cereals, and sweets consumed in Italy assessed by three different in vitro assays[J]. Molecular nutrition & food research, 2006, 50(11): 1030-1038.
Related Products:
BC1320/BC1325 Hydroxyl Radical Scavenging Capacity Assay Kit
BC1330/BC1335 Plant Flavonoids Assay Kit
BC1340/BC1345 Plant Total Phenol (TP)Assay Kit
BC1350/BC1355 Plant Proanthocyanidins Assay Kit


-scaled-400x400.jpg)
Reviews
There are no reviews yet.