Product Overview:
Dithiothreitol (DTT) is a reagent used to reduce protein S-S bonds. In biochemical research, it is a thiol-protecting agent, a specific enzyme inhibitor, and used to measure enzyme activity. It is also used in the preparation of SDS-PAGE sample buffer.
Preparation:
Dissolve 3.09g DTT in 20ml 0.01mol/L sodium acetate solution (pH5.2), filter and sterilize, then aliquot into 1ml vials and store at -20℃.
CAS Number:
Appearance:
White needle or white granular
Purity:
≥99%
Dithiothreitol Storage Conditions:
2-8℃
Shelf Life:
Four years
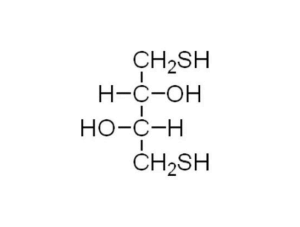
What is Dithiothreitol (DTT)?
Dithiothreitol (DTT) is a small molecule organic that can reduce agents with the chemical formula C4H10O2S2.
It is a linear molecule in the reduced state and becomes a six-membered ring structure containing a disulfide bond after oxidation.
The name dithiothreitol is derived from erythritol (a four-carbon sugar). The isomer of DTT is dithioerythritol (DTE), which is a C3-epimer of DTT.
What should you know about Dithiothreitol storage?
The General storage conditions for DTT powder should be stored at -20℃. 1M DTT solution can be stored at -20℃ for more than 2 years.
DTT is relatively unstable because of its easy oxidation by air, however, its shelf life can be extended by freezing or handling under an inert gas.
Since the nucleophilicity of protonated sulfur is lower, the effective reducing power of DTT also decreases with decreasing pH; Tris(2-carboxyethyl)phosphine HCl (TCEP hydrochloride) can be used as an alternative to DTT under low pH conditions, and it is also more stable than DTT.
What are the applications of DTT?
- It is used as a reducing and deprotection agent for thiolated DNA. The thiolated DNA terminal sulfur atoms tend to form dimers in solution, especially in the presence of oxygen. This dimerization greatly reduces the efficiency of certain coupling reaction experiments (such as the immobilization of DNA in biosensors). Adding DTT to the DNA solution and removing it after a period of reaction can reduce the dimerization of DNA.
- It is used to reduce disulfide bonds in proteins. It can be used to prevent the formation of intramolecular or intermolecular disulfide bonds between cysteine residues in proteins. However, DTT is generally unable to reduce disulfide bonds embedded in the protein structure (not accessible to solvent). The reduction of such disulfide bonds usually requires denaturation of the protein (heating at high temperature or adding denaturants such as 6M guanidine hydrochloride, 8M urea, or 1% SDS).
Conversely, the depth of embedding can be determined based on the difference in the rate of disulfide bond reduction in the presence of DTT.
Why DTT is a strong reducing agent?
DTT is a very strong reducing agent, and its reducing power is largely due to the conformational stability of its oxidized six-membered ring (containing a disulfide bond).
At pH 7, its redox potential is -0.33 volts.
Among them, the intermediate state formed in the reaction is very unstable, because the second thiol group on DTT tends to combine with the oxidized sulfur atom, so the intermediate state quickly transforms into the cyclic oxidized structure of DTT, thereby completing the reduction of the disulfide bond.
The reducing ability of DTT is affected by pH and only works when the pH is greater than 7. This is because only the deprotonated thiolate anion (-S-) is reactive, while the thiol (-SH) is not reactive; the pKa of mercaptan is usually about 8.3.
What are the hazards of DTT to the human body?
Dithiothreitol (DTT) is a small molecule organic reducing agent that emits an unpleasant odor. It can be harmful to health if inhaled, swallowed, or absorbed through the skin. Exposure to DTT can cause several health problems, including:
- Skin and eye irritation: DTT can irritate the skin and eyes upon contact. Symptoms may include redness, itching, burning, and watering eyes.
- Respiratory irritation: Inhalation of DTT dust or mist can irritate the respiratory tract, causing coughing, sneezing, shortness of breath, and wheezing.
- Skin sensitization: Prolonged or repeated exposure to DTT may lead to skin sensitization, where the skin becomes hypersensitive to DTT, causing an allergic reaction upon further contact. This can manifest as a rash, blisters, or swelling.
Safety precautions:
- Wear gloves and eye protection when handling DTT, especially in solid or high-concentration forms.
- Work in a well-ventilated fume hood to avoid inhalation.
- Avoid contact with skin and eyes.
- Wash hands thoroughly after handling DTT.
- Dispose of DTT waste according to local regulations.
Conclusion
DTT is a valuable tool in biological research, but it is important to handle it with care to avoid potential health hazards. By following the recommended safety precautions, you can minimize the risks associated with using DTT.
